The Open Serialization Communication Standard Working Group (Open-SCS) announced the accelerated development of an industrial interoperability standard to focus on healthcare packaging serialization interoperability across the plant’s equipment and systems and between the supply chain.
The Steering Committee of the Open Serialization Communication Standard Working Group (Open-SCS) announced the accelerated development of an industrial interoperability standard and associated requirements templates by the end of 2015 to focus on health care packaging serialization interoperability across a plant’s equipment and systems and between the supply chain systems.
Since the 2012 patent cliff, generic products now make up 80% of the global health care market and are a primary counterfeiting target where:
- Counterfeit drugs flood the market and generate ~$75B USD Revenue annually
- The ‘counterfeit industry’ is estimated to grow 20% annually
- In some countries, counterfeit drugs constitute as much as 70% of total drug supply.
McKinsey & Company states, “Global standards could enable substantial patient safety benefits and enable total health care cost reduction of $40 to 100 billion USD.”
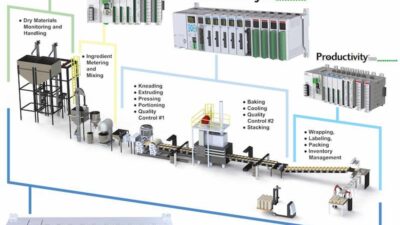
In September 2014, Open-SCS founding members first gathered in Frankfurt, Germany as the Open Architecture for Track & Trace Group to specifically address the lack of solution standardization for compliance to health care packaging serialization regulations. More than 80 health care manufacturers, solution providers, suppliers, and consulting companies contributed to this roundtable. The contributors worked on developing inter-plant serialization solutions to improve the deployment efficiency and high cost of compliance to the aggressive regulations.
Interoperability standards, such as OPC-UA, ISA-95/88/B2MML, EPC-IS, PackML, and others, cover most requirements, but there are gaps, suggested Charlie Gifford, executive director of the Open-SCS Group. "A single standard interoperability implementation will sufficiently cover the entire requirement for health care serialization compliance," Gifford said.
Serialization legislation from many countries dealing with the global healthcare counterfeiting crisis requires immediate serialization and aggregation of products from the manufacturer to the patient. This requires that production floor and warehouse equipment and systems are able to exchange information with the manufacturers’ supply chains and the patients’ support systems. The Open-SCS scope provided a blueprint on how these data exchanges can meet the following goals:
- Define and simplify the base roles for each actor in the data flow to supply chain.
- Define the communication protocols used for each connection point.
- Enable greater flexibility of the serialization architecture available to the industry.
- Substantially reduce integration cost and product delays from different vendors.
With all health care providers and vendors working together, the Open-SCS plans to release their standard and implementation specifications by the end of 2015.
OPC Foundation
www.opcfoundation.com
– Edited by Chris Vavra, production editor, Control Engineering, CFE Media, [email protected].