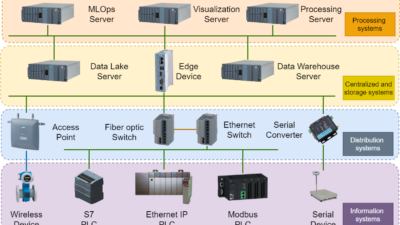
An Industrial Internet of Things (IIoT)-enabled global process validation system with advanced process control (APC) capabilities can accelerate manufacturing efficiency, production capacity and reduce production cycle time for the COVID-19 vaccine rollout.
As the COVID-19 pandemic is hitting the second wave across many nations, soaring up the total no. of COVID-19 positive cases around the globe, the world is yet again facing major healthcare and life sciences challenges, such as non-availability of hospital beds, scarcity of medical and paramedical staff and shortage of medication, medical devices and disposable medical equipment, etc.
On one hand, the world eagerly awaits vaccines to be successfully developed & tested by biopharma companies that can prevent public from getting infected by the deadly virus while ensuring utmost patient safety and on the other hand the FDA and pharmaceutical companies are working extensively to address the sudden shortage [1] of various drugs in the market including drugs related to COVID-19 caused due to interruptions in the manufacturing and supply chain caused by the pandemic and surge in demand for COVID-19 related treatment drugs.
An Industrial Internet of Things (IIoT)-enabled global process validation system with advanced process control (APC) capabilities can be a game changer in accelerating the manufacturing efficiency, production capacity and reducing production cycle time for chemical drugs and biologics to ensure availability of drugs while maintaining high process validation (PV) compliance that assures maximum quality ensuring patient safety.
Process validation and the role of IoT analytical capabilities
As per the World Health Organization (WHO), process validation [2] is the collection and evaluation of data, from the manufacturing process design stage through commercial production, which establishes scientific evidence a manufacturing process is capable of consistently delivering quality products that meet the product’s predetermined specifications and quality attributes. Process validation not only offers assurance a process is protected against sources of variability that could affect production quality, but also proves that in spite of any unavoidable variations in operational parameters through the necessary scale up of the production process, including new facilities and equipment, the final product quality characteristics are not compromised.
As per the US Food and Drug Administration’s guidance, there are three stages of process validation:
- Stage 1 – Process design: A systematic approach to development that begins with predefined objectives and emphasizes product and process understanding and process control, based on sound science and quality risk management
- Stage 2 – Process qualification: The process design is evaluated to determine if it is capable of reproducible commercial manufacture. This stage has two elements: (1) design of the facility and qualification of the equipment and utilities and (2) process performance qualification (PPQ). This should be completed before release of commercial lots.
- Stage 3 – Continued process verification (CPV): Collection and analysis of end-to-end production components and processes data to ensure product outputs are within predetermined quality limits assuring that processes are in a constant state of control, thus certifying final product quality.
Stage 3, the continued process verification (CPV) of process validation system plays the most important role in ensuring drug quality in a commercial scale production as it leverages statistical measures to determine process performance which in turn determines the batch quality.
Its in-built statistical process control (SPC) capabilities leverage data collected from manufacturing execution system (MES), or supervisory control and data acquisition (SCADA) and distributed control systems (DCS) connected to the manufacturing equipment for monitoring and minimizing deviations from the validated process. However, the scope of such a process verification system is limited to basic statistical process control charts of the batch data which lack the capability of dealing with the high speed IoT sensor data to optimize processes in real-time to maximize quality and minimize production cycle-time.
In order to improve the overall manufacturing efficiency in terms of drug quality and plant performance, the process validation system should be enhanced with APC capabilities that leverage big-data and advanced analytics driven IoT solutions. This is needed to scrutinize massive amounts of sensor data being collected by their smart machines and provide real-time predictive and prescriptive process improvement opportunities. Thus, IoT-enabled process validation system with APC capabilities have the potential to play a significant role in optimizing processes in real-time to achieve high drug quality.
Patents and global PV system for COVID-19 vaccines
Once a biopharma company succeeds in developing and testing COVID-19 vaccine, they would soon be applying for FDA drug approval for commercial production. However, the traditional approach to commercial rollout i.e., strict patents; is not going to work in case of COVID-19 vaccine. This pandemic is too big and important for any single company to be allowed to hold onto the manufacturing process rights. It is also almost impossible for a single company to produce vaccines that can cater to global demand.
Instead, it seems that once a vaccine is approved, its manufacturing will be licensed and outsourced to other biopharma manufactures or biopharma contract manufacturing organizations (CMOs) around the world for mass production. This, however, makes it difficult for the parent company or a regulatory authority such as FDA to monitor and govern the manufacturing processes followed across licensed companies which is essential to ensure the production quality of the vaccine.
Hence, in order to accelerate the global production rollout while maintaining high degree of compliance with the originally validated process, an overarching consolidated process validation system with APC capabilities is essential. It consolidates data from all the licensed companies’ production systems such as MES, laboratory information systems (LIMS), electronic batch record system (EBRS) etc., and provides predictive and prescriptive process improvement insights. This consolidated system not only helps in a quicker replication of the original process validation system by the licensed biopharma companies and CMOs, which directly reduces initial set-up time. It also helps the patent–holding biopharma company and the regulatory bodies such as FDA or WHO to govern and ensure a high degree of international quality and safety standards are met with minimum delays and errors.
In figure 2, consider Biopharma-A and Biopharma-B who have successfully developed COVID-19 vaccines, their certified process validation systems can be replicated in the “COVID-19 global process validation system” under two separate instances. These instances can then be then leveraged by other biopharma/CMOs for scaling-up vaccine production globally to ensure high quality.
Advanced process control (APC) capabilities in a PV system
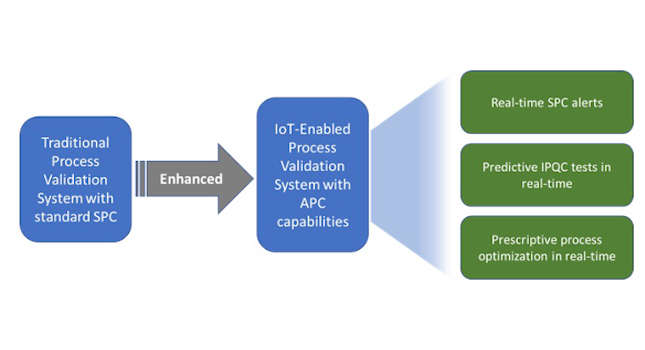
A traditional process validation system with statistical process control (SPC) capabilities keeps track of all types of activities that take place during manufacturing operations and notifies any process deviations to the quality control team using descriptive analytics.
The advanced process control (APC) driven process validation system takes into consideration all the significant parameters influencing drug quality such as raw materials, process parameters, asset parameters, environment parameters, etc [5]. It also applies advanced analytics techniques such as diagnostic, predictive and prescriptive analytics to identify quality leakages and their root causes and determines corrective and preventive actions (CAPA) and provides real-time process optimization insights for improving manufacturing quality of the current and future production batches.
For a better demonstration of APC capabilities of a desired IoT-enabled process validated system, we have considered the “Pharmaceutical Tablet Formulation manufacturing” use case in this paper. The proposed use case IoT solution capabilities can also be extended to “vaccines” manufacturing due to the fundamental similarities between chemical drug manufacturing and biologics drug manufacturing processes such as – the nature of in-process quality control (IPQC) tests throughout upstream and downstream processes, similar production systems such as MES & LIMS and most importantly compliance with the regulatory requirements of FDA’s cGMP practices.
Use case: Process optimization for tablet formulation manufacturing
A typical “tablet formulation” manufacturing process has multiple unit operations such as milling, mixing, blending, discharge or compression, coating, etc.. It also includes multiple in-process quality control (IPQC) tests [6] that are carried out by the quality team on the processed output at the end of every unit operation such as testing for “content uniformity,” “lending homogeneity,” “dissolution rate,” etc.
Since these IPQC tests are executed separately in the laboratory environment on the intermediate and finished samples collected from the batch under production, the quality control experts end up spending significant amount of their time to accurately determine if the IPQC tests meet all the batch acceptance criteria or not.
For example, a “dissolution” IPQC test involves placing each tablet from the sample into a dissolution medium that simulates human digestive fluids and record the percentage of dissolution of the tablet over a duration of 1 hour. In case the actual dissolution rate is higher than the minimum acceptable dissolution rate, the batch will be accepted or else it will be rejected. Such rejected batches not only lead to material loss in the form of scrap but also lead to production delays due to rework associated with a new batch production.
In case a detailed root cause analysis (RCA) and corrective and preventive actions (CAPA) are not implemented between the failed batch and the new batch under production, there is a high probability the new batch may also fail the impending IPQC tests. This means the quality team would be investing considerable amount of their time on conducting the IPQC tests on the new batch which are bound to fail leading to high scrap, rework and further production delays.
Over the years, statistical process control (SPC) [7] has played a crucial role in monitoring, measuring and controlling drug quality during the manufacturing process. However, it is primarily known for averting a few immediate defects, and it lacks the ability to provide predictive and prescriptive insights for continuous process improvement to improve quality and reduce production cycle time.
For example, in figure 4, the standard SPC alerts generated during each unit operation may have limited scope for real-time in-process decision making required to improve overall quality. Therefore, all applicable IPQC tests are performed by default to determine if the processed output at the end of each unit process will lead to batch acceptance or rejection.
Figure 5: Tablet formulation manufacturing with streaming analytics and predictive analytics. Courtesy: Industrial Internet Consortium[/caption]
While quality prediction ensures the low quality batches are discarded in advance reducing batch cycle time, the ultimate benefit of advanced analytics lies in not only predicting if IPQC tests will be leading to a batch acceptance or rejection but also prescribing optimum control settings for the subsequent unit operation’s process parameters, asset parameters and ambient parameters within the validated state so adjusting these parameter values will result in favorable IPQC tests ensuring overall batch acceptance.
This can be achieved by leveraging APC capabilities which is a combination of:
- Real-time SPC alerts for validated process compliance;
- IPQC prediction for predicting batch quality;
- Prescriptive process optimization for improving quality.
For example, in figure 6, if both predicted IPQC1 and actual IPQC1 test pass after operation-1 milling, then before the operation2-blending commences, IPQC2 test is predicted based on the results obtained after the actual IPQC1 test, i.e. Actual IPQC1 test result becomes one of the inputs to the predicted IPQC2 test. If this predicted IPQC2 before the Operation2-Blending fails, then APC prescribes adjusted parameter settings for Operation 2–Blending within the validated limits, so the “Operation 2-Blending” results in a positive predicted and eventually actual IPQC2 test results. Thus, this real-time process optimization minimizes overall batch rejection and improves manufacturing efficiency while ensuring the validated process is not compromised.
In a commercial manufacturing setup, the IIoT solutions such as IoT-enabled process validation system with APC capabilities can be successfully implemented in two ways:
- Revalidating the entire process with APC embedded process automation and establishing a new validated process; and
- Creating a parallel path for manual decision making that does not interfere with the existing validated process
In both these cases, it is essential to ensure the trustworthiness of the overall production system is not compromised. Industrial IoT systems are complex by nature as they are comprised of many systems and subsystems of both operations technology (OT) and information technology (IT) world, it is critical the proposed IoT-enabled process validation system is designed to ensure high trustworthiness is achieved at an individual system level and upon integration with other systems.
Thus, a trustworthy IoT-enabled global process validation system with APC capabilities, that has the potential to connect to disparate data sources and analyze historical and real-time operational, asset and environmental batch data and that offers a wide range of analytical capabilities from basic descriptive and diagnostic analytics to more advanced predictive and prescriptive analytics will play a significant role in supporting life sciences manufacturers and their licensed partners in rolling out Covid-19 vaccines and other related treatment drugs in order to meet the global demand.
Conclusion
IoT-led global process validation system with APC capabilities not only ensure that the patent company and regulatory bodies have high control and governance over the license companies’ compliance with the originally validated process in real time, but also its advanced analytical capabilities reduce production cycle time by predicting and minimizing IPQC test requirement and improve manufacturing batch quality by optimizing processes in real-time.
Ramya Mopidevi, senior industry consultant, SAS Institute. This article originally appeared in the IIC Journal of Innovation. The Industrial Internet Consortium (IIC) is a CFE Media content partner. Edited by Chris Vavra, web content manager, Control Engineering, CFE Media and Technology, [email protected].
References:
[1] https://www.fda.gov/drugs/drug-safety-and-availability/drug-shortages
[2] https://www.fda.gov/files/drugs/published/Process-Validation–General-Principles-and-Practices.pdf
[4] https://thewire.in/law/covid-19-crisis-wto-intellectual-property-vaccine-public-health
[5] https://www.pharmamanufacturing.com/articles/2005/337/
[6] https://zenodo.org/record/2527439/files/11.pdf
[7] https://ispe.org/pharmaceutical-engineering/ispeak/supporting-pharma-innovation-through-analytics